

|
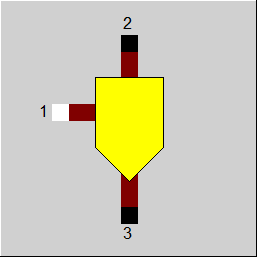
Line connections |
|
|
|
1 |
Inlet |
|
|
2 |
Outlet |
|
|
3 |
Branching with mass flows of the selected components |
|
General User Input Values Physics Used Displays Example
Component 52 enables the separation of the material flows of a fluid (gaseous, liquid or solid). The following can be modelled, for instance, with this component:
The proportionate extraction of a material X is described by the specification value JX, whereby X is the short name of the material. The materials that can be extracted depend upon the fluid type. See the chapter on Pipe Overview for more details.
Alternatively, the composition of the separated fluid can be specified externally (by a component 33, start value). In this case, it is required that the substances that have to be withdrawn are available in the inlet flow in sufficient quantities.
This component allows a transition between different material tables, even with different zero points for the enthalpy. A transition to the 2-phase material library is possible for the fluids LibNH3, LibCO2 and Water. For user-defined fluids, it is not possible.
Example for the application: separation of CO2 from the flue gas with liquidizing afterwards
Note the behaviour of this component when you separate fluids with phase transitions: when you separate only one phase, component 52 separates as much from this fluid as corresponds of the specified phase at the inlet of component 52. Nevertheless, at the outlets of the components, there will be a new phase equilibrium of both phases according to the thermodynamical quantities and the partial pressures in the outlet pipes.
To facilitate the modeling of chemical processes, the modes FM = 4 and FM = 5 have been implemented to allow to define the ratio of the substances at the outlet. Here the specification values J.. are not interpreted as separation rates but as the relative ratio of the substances among each other that is to be achieved on the outlet line. At FM = 4, the mass fractions are specified here, and at FM = 5 the molar fractions.
The separation quantity is defined by the specification value RR (reaction rate). For RR = 1, as much as possible is separated, i.e. so much that nothing is left of (at least) one substance.
Until Release 16 Patch 2, substances with a content of less than 10^-10 were ignored. However, this restriction proved to be unnecessary and was therefore lifted.
The total mass flow of line 3 results from the junction rates Ji (0<=Ji<=1, i stands for the components) and the weight parts X1i=M1i/M1 of line 1
M3= S[X1i * Ji] * M1
The portion at the line 3 is calculated analog to
X3i = X1i * Ji * M1/M3
The relations for mass flow and the components at line 2 result in
M2= S[X1i * (1-Ji)] * M1
X2i = X1i * { 1-Ji } * M1/M2
The pressure is assumed to be constant
If FSPECH=0, then (T1 is specified)
T3=T1, T2 from energy balance
If FSPECH=1, then (T2 is specified)
T1=T2, T3 from energy balance
If FSPECH=2, then (T1 is specified)
T3=T2=T1, without taking into account the energy balance
The enthalpies result from the total of the specific enthalpies Hi at the temperatures and the components Xi result in
H2= S[Hii * X2i]
H3= S[Hii * X3i]
The branching mass flow 3 must be specified (e.g. through the component 33, pastille) or else it must be known in the system by other means.
The branching mass flow 3 must be specified via a characteristic line, which specifies the branching behaviour depending upon the incoming mass flow.
The branching mass flow 3 is specified as a portion in the incoming mass flow. This relation does not change for the off-design mode.
The extracted wetness results from the specification of the portion, by which the available wetness in the wet steam is to be reduced.
This component determines, whether water is present in air, flue gas, fuel gas, coal or crude gas as a result of the partial pressures. It can be defined, which portion of the available wetness is to be reduced.
|
FM |
Mass flow distribution =2: M1 or M3 given |
| RR | Used with FM=4 or FM=5 only: Reaction rate |
|
FSPECH |
Handling of energy balance |
|
FADAPT |
Flag for using the adaptation polynomial ADAPT/ adaptation function EADAPT =0: Not used and not evaluated
= -1000: Not used, but EADAPT evaluated as RADAPT (Reduction of the computing time) |
|
EADAPT |
Adaptation Function |
|
JN2 |
N2 fraction |
|
JO2 |
O2 fraction |
|
JCO2 |
CO2 fraction |
|
JH20G |
Water fraction (gaseous) |
|
JH20L |
Water fraction (liquid) |
|
JSO2 |
SO2 fraction |
|
JAR |
Argon fraction |
|
JCO |
CO fraction |
|
JCOS |
COS fraction |
|
JH2 |
H2 fraction |
|
JH2S |
H2S fraction |
|
JCH4 |
CH4 fraction |
|
JHCL |
HCL fraction |
|
JETH |
Ethane fraction |
|
JPROP |
Propane fraction |
|
JBUT |
n-Butane fraction |
|
JPENT |
n-Pentane fraction |
|
JHEX |
n-Hexane fraction |
|
JHEPT |
n-Heptane fraction |
|
JACET |
Acetylene fraction |
|
JBENZ |
Benzene fraction |
|
JC |
C fraction |
|
JH |
H fraction |
|
JO |
O fraction |
|
JN |
N fraction |
|
JS |
S fraction |
|
JCL |
CI fraction |
|
JASH |
Ash fraction (not gaseous) |
|
JASHG |
Ash fraction (gaseous) |
|
JLIME |
Lime (Ca(OH)2) fraction |
|
JCA |
Elemental calcium fraction |
|
JH2OB |
chemically bonded water fraction (Water in fuel) |
|
JNO |
NO-fraction |
|
JNO2 |
NO2-fraction |
|
JNH3G |
fraction of gaseous NH3 |
|
JNH3L |
fraction of liquid NH3 |
|
JMG |
Elemental magnesium (Mg) fraction |
|
JMETHL |
fraction Methanol |
|
JCACO3 |
CaCO3 fraction |
|
JCAO |
CaO-fraction |
|
JCASO4 |
CaSO4-fraction |
|
JMGCO3 |
MgCO3-fraction |
|
JMGO |
MgO fraction |
|
JOCT |
n-Octane fraction |
|
JNON |
n-Nonane fraction |
|
JDEC |
n-Decane fraction |
|
JDODEC |
n-Dodecane fraction |
|
JIBUT |
Isobutane (2-Methylpropane) fraction |
|
JIPENT |
Isopentane (2-Methylbutane) fraction |
|
JNEOPENT |
Neopentane (2,2-Dimethylpropane) fraction |
|
J22DMBUT |
Neohexane (2,2-Dimethylbutane) fraction |
|
J23DMBUT |
2,3-Dimethylbutane fraction |
|
JCYCPENT |
Cyclopentane fraction |
|
JIHEX |
Isohexane (2-methylpentane) fraction |
|
J3MPENT |
3-Methylpentane fraction |
|
JMCYCPENT |
Methylcyclopentane fraction |
|
JCYCHEX |
Cyclohexane fraction |
|
JMCYCHEX |
Methylcyclohexane fraction |
|
JECYCPENT |
Ethylcyclopentane fraction |
|
JECYCHEX |
Ethylcyclohexane fraction |
|
JTOLUEN |
Toluene (toluol, methylbenzene) fraction |
|
JEBENZ |
Ethylbenzene fraction |
|
JOXYLEN |
ortho-Xylene (1,2-dimethylbenzene) fraction |
|
JCDECALIN |
cis-Decalin (decahydronaphthalene) fraction |
|
JTDECALIN |
trans-Decalin (Decahydronaphthalene) fraction |
|
JETHEN |
Ethene (ethylene) fraction |
|
JPROPEN |
Propene (propylene) fraction |
|
J1BUTEN |
1-Butene fraction |
|
JC2BUTEN |
cis-2-Butene fraction |
|
JT2BUTEN |
trans-2-Butene fraction |
|
JIBUTEN |
Isobutene (2-Methylpropene) fraction |
|
J1PENTEN |
1-Pentene fraction |
|
JPROPADIEN |
Propadiene (allene) fraction |
|
J12BUTADIEN |
1,2-Butadiene (methylallene) fraction |
|
J13BUTADIEN |
1,3-Butadiene (vinylethylene) fraction |
|
JETHL |
Ethanol fraction |
|
JCH3SH |
CH3SH (methanethiol, methylmercaptan) fraction |
|
JHCN |
Hydrogen cyanide (prussic acid) fraction |
|
JCS2 |
Carbon disulfide fraction |
|
JAIR |
Air fraction |
|
JHE |
Helium fraction |
|
JNE |
Neon fraction |
|
JKR |
Krypton-fraction |
|
JXE |
Xenon-fraction |
|
JN20 |
Dinitrogen monoxide (laughing gas) fraction |
|
JCO2L |
CO2 (liquid)-fraction |
|
JH2OL |
Water (liquid)-fraction |
|
JNH3L |
NH3 (liquid) fraction |
|
DTMAX |
Maximum permissible temperature deviation (taking into account the energy balance) |
Input: 0<=Ji<=1 , i stands for the components
Generally, all inputs that are visible are required. But, often default values are provided.
For more information on colour of the input fields and their descriptions see Edit Component\Specification values
For more information on design vs. off-design and nominal values see General\Accept Nominal values
|
All cases |
||
|
p2 = p1 (1) p3 = p1 (2) M3i= X1i *Ji*M1 M3 = S(M3i) (5) X3i = X1i * Ji * M1/M3 M2 = M1 - M3 (6) X2i = X1i * { 1-Ji } * M1/M2 X3i from the mass balance H2 = S [ Hi * X2i ] (3) H3 = S [ Hi * X3i ] (4) FADAPT = 0: ADAPT Not used and not evaluated FADAPT = 1 : ADAPT = Factor of all Jxx (i.e. all separation rates are multiplied with the same factor) FADAPT = 1000 : Not used, but ADAPT evaluated as RADAPT (Reduction of the computing time) FADAPT = -1: EADAPT = Factor of all Jxx (i.e. all separation rates are multiplied with the same factor) FADAPT = -2: EADAPT = Separation rate for all Jxx (With the use of subst_id, all separation rates can be different) FADAPT = -1000 : Not used, but EADAPT evaluated as RADAPT (Reduction of the computing time) |
||
If, for instance, you want to separate half of the water and all of the CO2 and all of the SO2, you can use the following EADAPT-code:
function evalexpr:REAL;
 |
Display Option 1 |